| <Prev Next> |
| For all states | 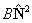 |
Rotation |
| S > 0 | 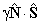 |
Spin-Rotation |
| Λ > 0, S > 0 | 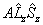 |
Spin-Orbit |
| S > 1/2 | 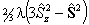 |
Spin-Spin |
| S > 3/2 | 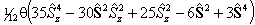 |
Spin-Spin |
| Π states | 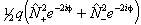 |
Λ doubling |
| Π states, S > 0 | 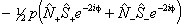 |
Λ doubling |
| Π states, S > 1/2 | 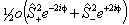 |
Λ doubling |
See the individual sections below for a more detailed discussion. Note that any operators not listed above (such as alternate forms of operators or higher powers of centrifugal distortion) can be generated using a perturbation diagonal in electronic state.
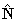 :
:
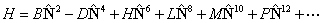
though formally, it should only involve the rotational angular
momentum of the nuclear framework, 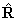 and thus be written:
and thus be written:
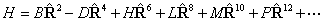
PGOPHER can use either
form, as controlled by the "RSquaredH" flag
at the molecule level. This flag affects many of the terms in the
Hamiltonian; for all the linear molecule terms read 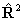 for
for 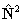 if
RSquaredH=True; to follow the IUPAC recommendations the
if
RSquaredH=True; to follow the IUPAC recommendations the 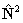 form must be used.
form must be used.
The reason both forms are in use is that each has some problems in
evaluation, as discussed by Brown et al, 1987. Strictly the
second form is correct, but full evaluation is not possible because of
the terms involving 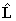 . Consider evaluating the
. Consider evaluating the 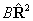 term:
term:
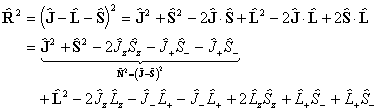
The diagonal terms are:
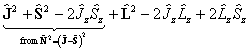
which becomes:
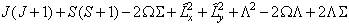
The 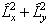 term must be
discarded, leaving:
term must be
discarded, leaving:
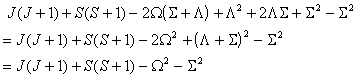
which is the form used. The equivalent expression in 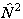 is:
is:
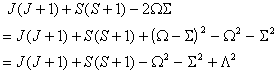
i.e. simply an additional Λ2 term compared to 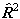 . The main practical difference is a shift
in the effective band origin of BΛ2
(so they are identical for Σ states), but it is not clear that one is
any more correct than the other as both ignore the term
. The main practical difference is a shift
in the effective band origin of BΛ2
(so they are identical for Σ states), but it is not clear that one is
any more correct than the other as both ignore the term 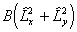 .
This latter term is only likely to be worth considering if isotope
shifts of vibrational band origins are required, and the former leads
to different definitions of the band origin, so it is worth checking
all published constants to see which is used. The matrix elements of
higher powers of
.
This latter term is only likely to be worth considering if isotope
shifts of vibrational band origins are required, and the former leads
to different definitions of the band origin, so it is worth checking
all published constants to see which is used. The matrix elements of
higher powers of 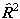 or
or 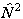 are evaluated by evaluating the matrix of
are evaluated by evaluating the matrix of
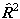 or
or 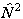 as above and taking the appropriate power of the matrix. This means
that the RSquaredH flag
will cause small changes will affect most of
the constants so, for example, B will change by 2Λ2D.
as above and taking the appropriate power of the matrix. This means
that the RSquaredH flag
will cause small changes will affect most of
the constants so, for example, B will change by 2Λ2D.
Note that off-diagonal terms:
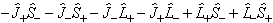
are normally discarded in both forms of the Hamiltonian as they only connect completely different electronic states so they are not normally important. They can be included as a perturbation if required (see Luncouple)
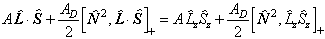
where:![[O,Q]+=OQ+QO](linham/pic033.gif)
For states of high multiplicity there are other terms that may need to be considered; see references in Brown et al, 1987.
For S > 1/2 the spin-spin term is required:
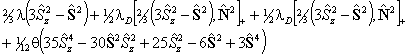
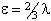 is also in use. The term in θ is
only applicable for states with S > 3/2; again see
references in Brown et al, 1987. Read
is also in use. The term in θ is
only applicable for states with S > 3/2; again see
references in Brown et al, 1987. Read 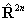 for
for 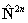 if RSquaredH=True.
if RSquaredH=True.
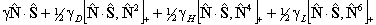
If RSquaredH=True, read 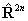 for
for 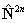 but
note the
but
note the 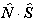 term is unchanged.
term is unchanged.
The IUPAC form is:
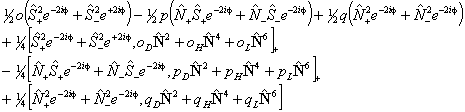
Of these terms q will contribute for any Π state, p requires S > 0 also and o will only contribute for S > 1/2. The e±2iφ terms are shorthand to ensure that the Λ-doubling operators only connect the two halves of a Π state:
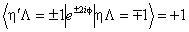
If RSquaredH=True, read 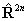 for
for 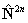 in
the centrifugal distortion terms, and leave the other operators
unchanged. There are alternative Λ doubling parameters, in use
including:
in
the centrifugal distortion terms, and leave the other operators
unchanged. There are alternative Λ doubling parameters, in use
including:
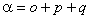
This arises naturally from expressing the Hamiltonian in terms of J,
rather than N as can be seen by making the replacement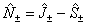 :
:

See Brown and Merer, 1979 for a discussion of this. This also
explains why under some circumstances only p + 2q is
determined rather than p and q individually. (A term
equivalent to this can be generated using perturbations.)